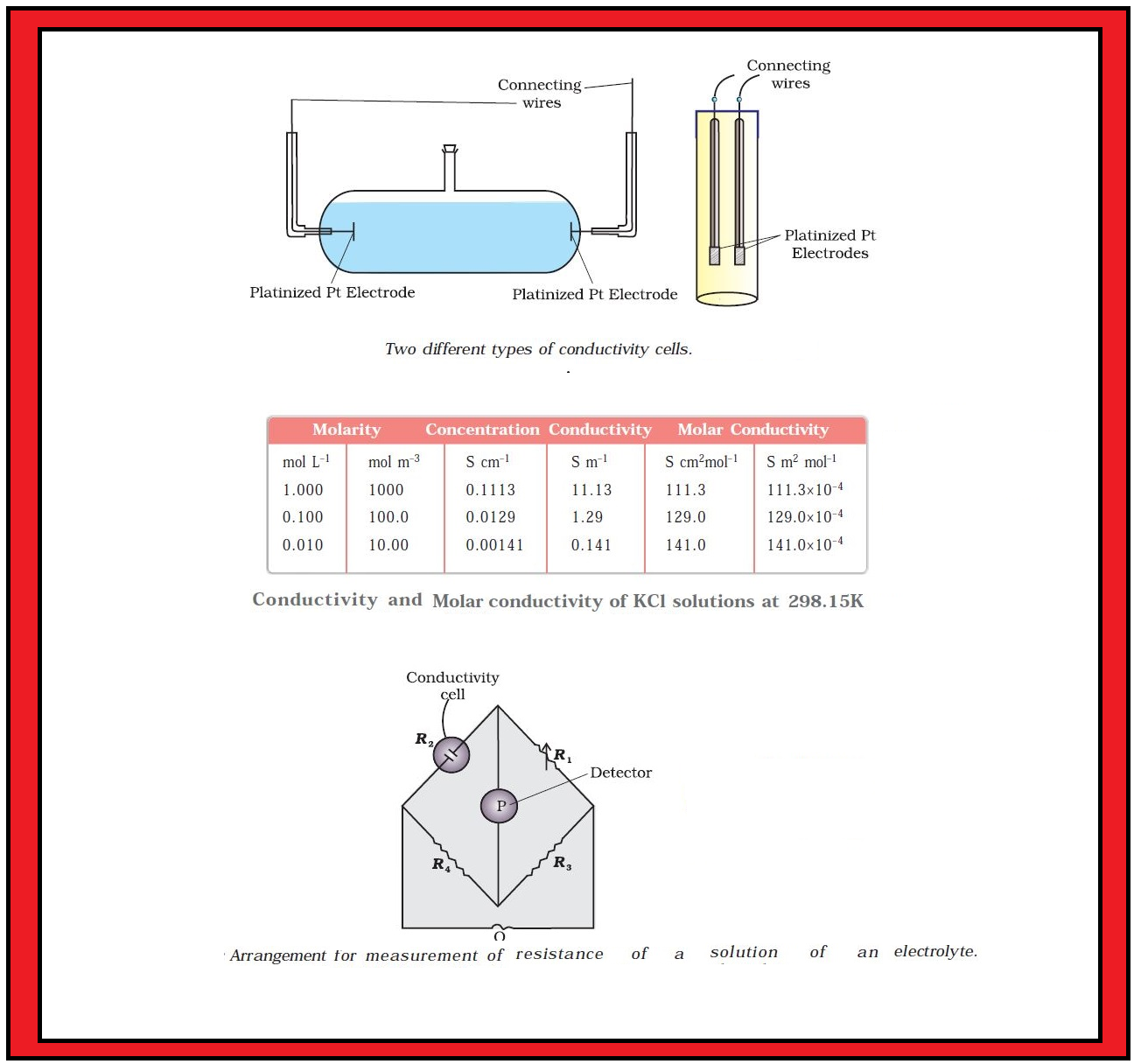
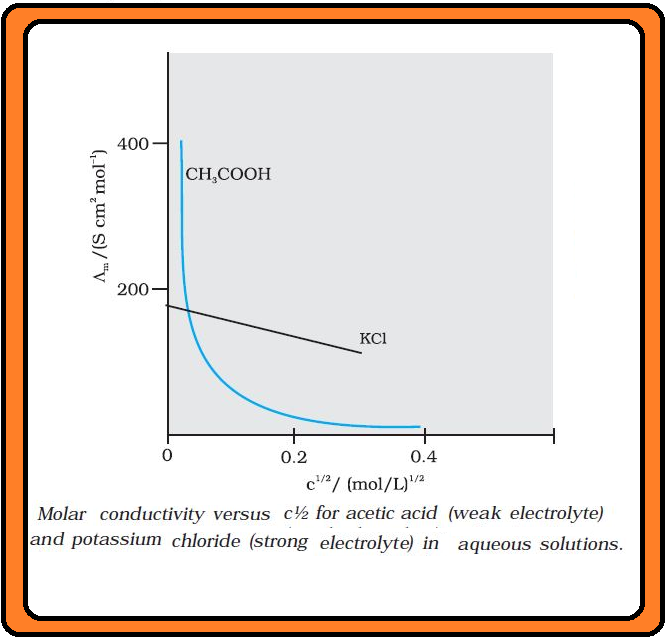
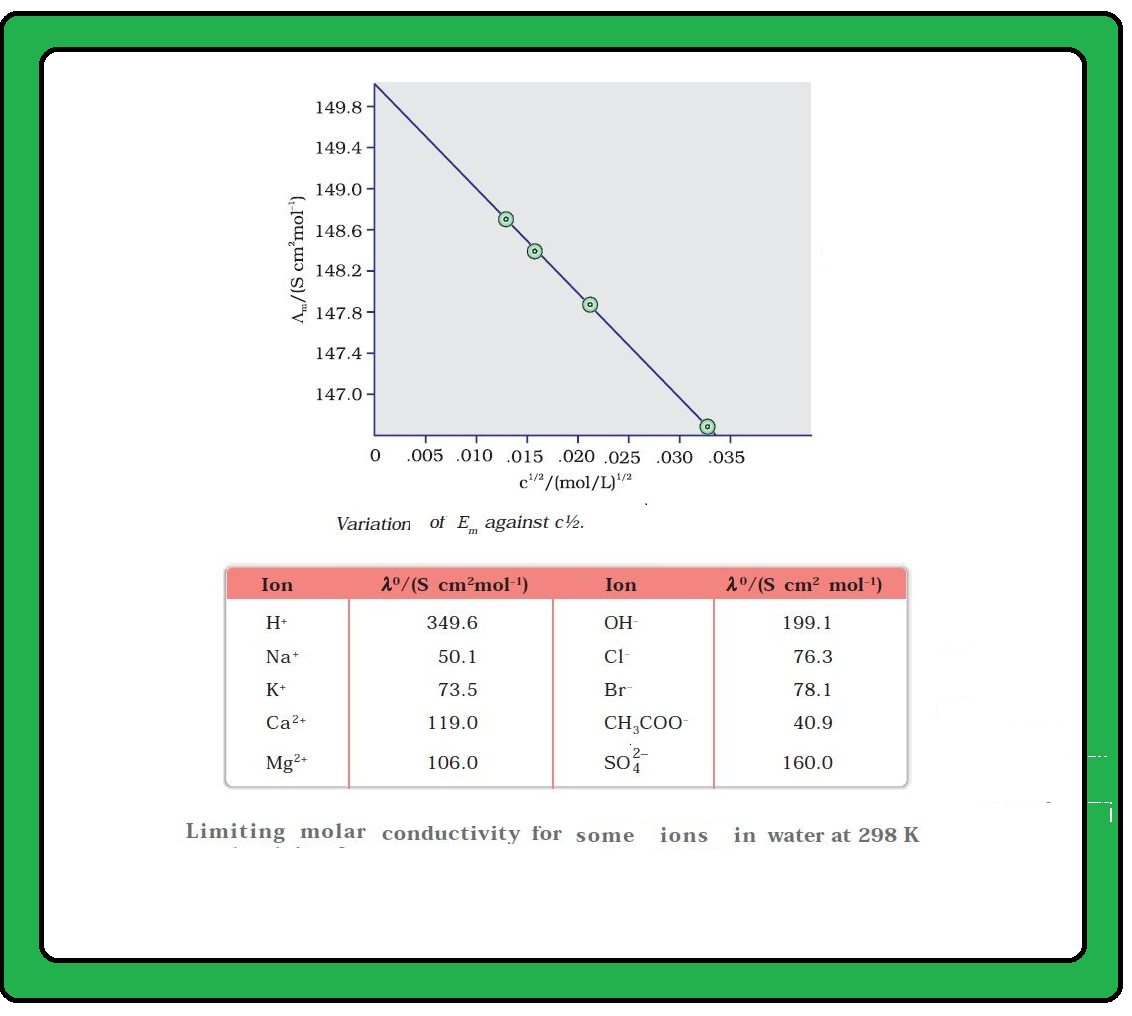
Conductance of Electrolytic Solutions :

First, we will define a few terms before we consider the subject of conductance of electricity through electrolytic solutions.
`color{green}("Resistance") :` The electrical resistance is represented by the symbol `‘R’` and it is measured in ohm `(Ω)` which in terms of `SI` base units is equal to `color{red}((kg m^2)/(s^3 A^2))`. It can be measured with the help of a Wheatstone bridge. The electrical resistance of any object is directly proportional to its length, `l`, and inversely proportional to its area of cross section, `A`. That is,
`color{red}(R prop l/A)` or `color{red}(R = rho l/A)` ............(1)
`color{green}("Resistivity") :` The constant of proportionality, `ρ` (Greek, rho), is called resistivity (specific resistance).
`=>` Its `SI` units are ohm metre `(Ω m)` and quite often its submultiple, ohm centimetre `(Ω cm)` is also used.
`=>` IUPAC recommends the use of the term resistivity over specific resistance.
`=>` The resistivity for a substance is its resistance when it is one metre long and its area of cross section is one `m^2`.
It can be seen that :
`color{red}(1 Ω m = 100 Ω cm & 1 Ω cm = 0.01 Ω m)`
`color{green}("Conductance") :` The inverse of resistance, `R`, is called conductance, `G`, and we have the relation :
`color{red}(G = 1/R = A/(rho l) = k A/l)` ..............(2).
`=>` The SI unit of conductance is siemens, represented by the symbol `‘S’` and is equal to `ohm^(–1)` (also known as mho) or `Ω^(–1)`.
`color{green}text(Conductivity) :` The inverse of resistivity, called conductivity (specific conductance) is represented by the symbol, `κ` (Greek, kappa).
`=>` IUPAC has recommended the use of term conductivity over specific conductance.
`=>` The SI units of conductivity are `S m^(–1)` but quite often, `κ` is expressed in `S cm^(–1)`.
`=>` Conductivity of a material in `S m^(–1)` is its conductance when it is `1 m` long and its area of cross section is `1 m^2`.
`=>` `color{red}(1 S cm^(–1) = 100 S m^(–1))`.
`=>` It can be seen from Table that the magnitude of conductivity varies a great deal and depends on the nature of the material.
`=>` It also depends on the temperature and pressure at which the measurements are made.
`=>` Materials are classified into conductors, insulators and semiconductors depending on the magnitude of their conductivity.
`=>` Metals and their alloys have very large conductivity and are known as conductors. Certain non-metals like carbon-black, graphite and some organic polymers* are also electronically conducting.
`=>` Substances like glass, ceramics, etc., having very low conductivity are known as insulators.
`=>` Substances like silicon, doped silicon and gallium arsenide having conductivity between conductors and insulators are called semiconductors and are important electronic materials.
`=>` Certain materials called superconductors by definition have zero resistivity or infinite conductivity.
`=>` Earlier, only metals and their alloys at very low temperatures (`0` to `15 K`) were known to behave as superconductors, but nowadays a number of ceramic materials and mixed oxides are also known to show superconductivity at temperatures as high as `150 K`.
`color{green}text(Electrical Conductance) :` Electrical conductance through metals is called metallic or electronic conductance and is due to the movement of electrons.
`=>` The electronic conductance depends on
(i) the nature and structure of the metal
(ii) the number of valence electrons per atom
(iii) temperature (it decreases with increase of temperature).
`=>` As the electrons enter at one end and go out through the other end, the composition of the metallic conductor remains unchanged. The mechanism of conductance through semiconductors is more complex.
We know that even very pure water has small amounts of hydrogen and hydroxyl ions `(~10^(–7M))` which give it very low conductivity `(3.5 × 10^(–5) S m^(–1))`. When electrolytes are dissolved in water, they furnish their own ions in the solution hence its conductivity also increases.
`color{green}("Ionic Conductivity") :` The conductance of electricity by ions present in the solutions is called electrolytic or ionic conductance.
`=>` The conductivity of electrolytic (ionic) solutions depends on :
(i) the nature of the electrolyte added
(ii) size of the ions produced and their solvation
(iii) the nature of the solvent and its viscosity
(iv) concentration of the electrolyte
(v) temperature (it increases with the increase of temperature).
Passage of direct current through ionic solution over a prolonged period can lead to change in its composition due to electrochemical reactions.
`color{green}("Resistance") :` The electrical resistance is represented by the symbol `‘R’` and it is measured in ohm `(Ω)` which in terms of `SI` base units is equal to `color{red}((kg m^2)/(s^3 A^2))`. It can be measured with the help of a Wheatstone bridge. The electrical resistance of any object is directly proportional to its length, `l`, and inversely proportional to its area of cross section, `A`. That is,
`color{red}(R prop l/A)` or `color{red}(R = rho l/A)` ............(1)
`color{green}("Resistivity") :` The constant of proportionality, `ρ` (Greek, rho), is called resistivity (specific resistance).
`=>` Its `SI` units are ohm metre `(Ω m)` and quite often its submultiple, ohm centimetre `(Ω cm)` is also used.
`=>` IUPAC recommends the use of the term resistivity over specific resistance.
`=>` The resistivity for a substance is its resistance when it is one metre long and its area of cross section is one `m^2`.
It can be seen that :
`color{red}(1 Ω m = 100 Ω cm & 1 Ω cm = 0.01 Ω m)`
`color{green}("Conductance") :` The inverse of resistance, `R`, is called conductance, `G`, and we have the relation :
`color{red}(G = 1/R = A/(rho l) = k A/l)` ..............(2).
`=>` The SI unit of conductance is siemens, represented by the symbol `‘S’` and is equal to `ohm^(–1)` (also known as mho) or `Ω^(–1)`.
`color{green}text(Conductivity) :` The inverse of resistivity, called conductivity (specific conductance) is represented by the symbol, `κ` (Greek, kappa).
`=>` IUPAC has recommended the use of term conductivity over specific conductance.
`=>` The SI units of conductivity are `S m^(–1)` but quite often, `κ` is expressed in `S cm^(–1)`.
`=>` Conductivity of a material in `S m^(–1)` is its conductance when it is `1 m` long and its area of cross section is `1 m^2`.
`=>` `color{red}(1 S cm^(–1) = 100 S m^(–1))`.
`=>` It can be seen from Table that the magnitude of conductivity varies a great deal and depends on the nature of the material.
`=>` It also depends on the temperature and pressure at which the measurements are made.
`=>` Materials are classified into conductors, insulators and semiconductors depending on the magnitude of their conductivity.
`=>` Metals and their alloys have very large conductivity and are known as conductors. Certain non-metals like carbon-black, graphite and some organic polymers* are also electronically conducting.
`=>` Substances like glass, ceramics, etc., having very low conductivity are known as insulators.
`=>` Substances like silicon, doped silicon and gallium arsenide having conductivity between conductors and insulators are called semiconductors and are important electronic materials.
`=>` Certain materials called superconductors by definition have zero resistivity or infinite conductivity.
`=>` Earlier, only metals and their alloys at very low temperatures (`0` to `15 K`) were known to behave as superconductors, but nowadays a number of ceramic materials and mixed oxides are also known to show superconductivity at temperatures as high as `150 K`.
`color{green}text(Electrical Conductance) :` Electrical conductance through metals is called metallic or electronic conductance and is due to the movement of electrons.
`=>` The electronic conductance depends on
(i) the nature and structure of the metal
(ii) the number of valence electrons per atom
(iii) temperature (it decreases with increase of temperature).
`=>` As the electrons enter at one end and go out through the other end, the composition of the metallic conductor remains unchanged. The mechanism of conductance through semiconductors is more complex.
We know that even very pure water has small amounts of hydrogen and hydroxyl ions `(~10^(–7M))` which give it very low conductivity `(3.5 × 10^(–5) S m^(–1))`. When electrolytes are dissolved in water, they furnish their own ions in the solution hence its conductivity also increases.
`color{green}("Ionic Conductivity") :` The conductance of electricity by ions present in the solutions is called electrolytic or ionic conductance.
`=>` The conductivity of electrolytic (ionic) solutions depends on :
(i) the nature of the electrolyte added
(ii) size of the ions produced and their solvation
(iii) the nature of the solvent and its viscosity
(iv) concentration of the electrolyte
(v) temperature (it increases with the increase of temperature).
Passage of direct current through ionic solution over a prolonged period can lead to change in its composition due to electrochemical reactions.